By Frangiska Mylona,
Back in 1976, a brand-new and promising drug was introduced to the pharmaceutical market. It is known by its brand name ”Mediator” and includes the substance Benfluorex, which was authorized to help patients with poorly controlled diabetes type-2 to decrease their weight by reducing their insulin resistance and improving their glycemic control. After that, it was prescribed by doctors and dispensed by pharmacists as an off-labeled drug, often for weight loss outside of the approved indications, sometimes due to patients’ insistent urging.
Off-labeled drugs refer to drugs administered for a different purpose than they are approved and indicated for. These prescriptions are difficult to track down because rarely the “off-labeled” phrase is written on the prescriptions nor do pharmacists know the exact reason they are called to give this drug to the patient.

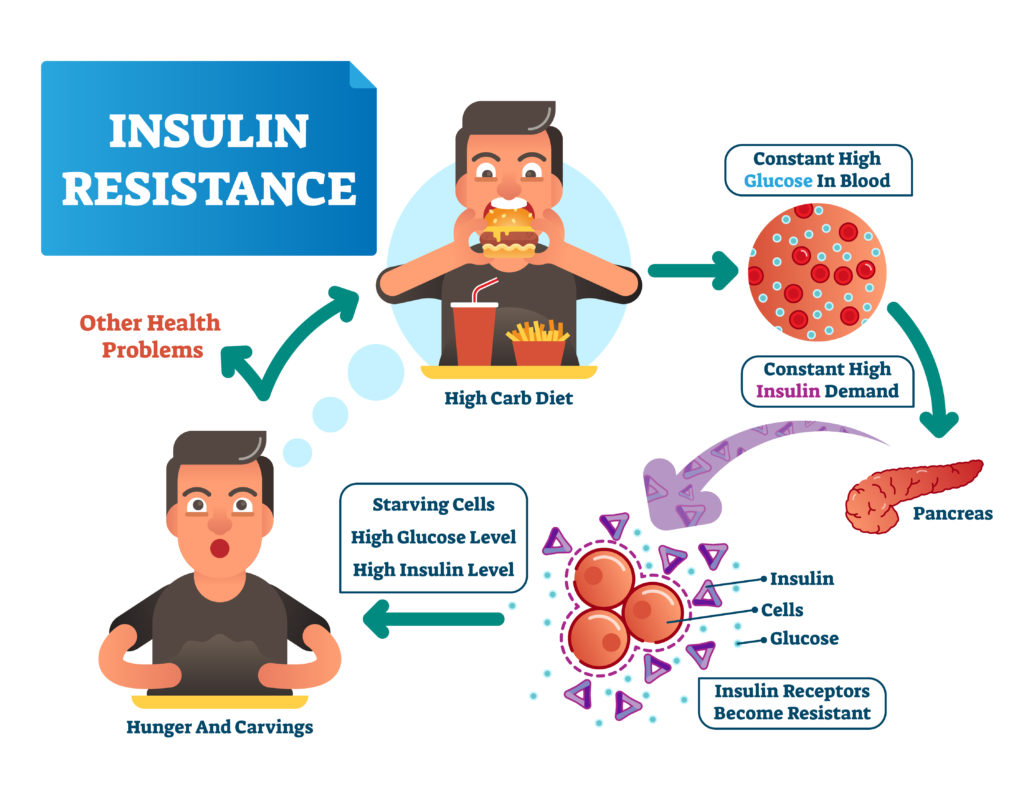
Diabetes type 2 is a form of chronic hyperglycemia caused by insulin resistance often progressing to insulin deficiency. Upon eating a meal, where glucose levels rise, insulin is produced by the endocrine cells of the pancreas, particularly by the β-cells of the islets of Langerhans. Its main functions are inhibiting lipolysis, facilitating triglyceride storage, and increasing glucose storage (as glycogen in the liver and in the muscles), therefore decreasing glucose levels in the blood. However, when there is increased insulin resistance by the body due to obesity, it can progress to diabetes type 2, as well as, atherosclerosis, kidney problems, or even stroke.
It was not until November 2009 that the Mediator drug was accused of causing heart valvular defects and removed from the French market.
The first suspicions about the severe adverse effects of the drug arose around 1986 to the late 1990s, stating that Benfluorex could increase the risks of pulmonary arterial hypertension and heart valve disease. Also, around the end of the previous century, in 1999, data emerged supporting that the Mediator drug was used as an off-labeled drug for weight loss surrendering its anorectic profile.
It is interesting that from 1990 till 1999 the Mediator’s anorectic profile was not officially reported in France, while the World Health Organization has listed the drug as an anorectic. Adding more to that, in 1995, France prohibited the mixing of Benfluorex with other anorexics in creating offhand drug preparations.
During the same period, Fenfluramine, a related drug to Benfluorex, was withdrawn from the market after some reports of inducing heart valve disease, pulmonary hypertension, and the development of cardiac fibrosis. The connection between Fenfluramine and Benfluorex is that after being metabolized by the human body, they give off the same active particular metabolite norfenfluramine that acts on specific receptors on the heart valves causing the proliferation of the cardiac fibroblasts especially on the tricuspid valve. However, Benfluorex would be retracted from the market years later than Fenfluramine.
Many started to doubt its anti-diabetic properties by questioning the drug’s overall benefits for diabetic patients. Furthermore, it was strongly believed that the immaculate advertisement of the drug was responsible for its success rather than its medical effects based on evidence.
The first cases of heart valvular problems appeared around 1998 to 1999, however, two important events occurred that confused and covered the unpleasant effects of the Mediator drug. First of all, around that time heart-valve diseases in anorectic patients were almost exclusively attributed to the rheumatic fever disease that causes heart-valvular problems as well. Secondly, the first case in France that was thought to be associated with this drug occurs in 1999 in Marseille, but due to dysfunctions within the French drug regulatory agency, the case was buried and then forgotten.
In Spain, in 2003, heart valvular defects were finally associated with Mediator drug. The valve lesions were of the same type as seen in Fenfluramine. This was the final straw, leading to the drug being withdrawn in Spain at that same time.
However, as mentioned above, the drug will not be withdrawn in France until 2009, where the years more and more shocking evidence about its lethal effects came to light. Specifically; in 2010 studies estimated that Mediator had caused 500 deaths through heart valve disease in France. Another study estimated that over 1300 such deaths occurred between 1976 and 2009 leading to a total of 6743 cases of heart valve disease and 1273 cases of pulmonary arterial hypertension reported in France between 1976 and 2015!
In 2014, the French administrative court accused Servier, the drug’s manufacturer company, of drug misconduct. The judgment was appealed in the Council in 2017 and in September 2019, the trial opens, while, in the same year, the victims of the Mediator drug that were either alive or dead finally got acknowledged and the demand for compensation gets stronger than ever.

From 2021 until now a cycle of counter-appeals has started. Specifically, the drug company was condemned to pay 2,7 million euros in 2021, although it was not found guilty of fraud or false information. This caused turmoil with the Paris legal authorities calling for a bigger fine. This was the trigger for the counter-appeal by the company that blamed the prosecution for extending unnecessarily the trial. This is continued till today, with many people, patients or relatives of diseased patients, fighting for their rights. A key person in this situation is Irène Frachon, a pulmonologist, that linked Mediator and the disorders she had observed. She suspected the effects of the drug for many years and she fought for the patients – especially women – who by desiring to lose weight took this anti-diabetic drug as an appetite suppressant.

The Mediator scandal is one of the many consequences of the false-body image that society and, in the later years, social media imposes on many people – especially women. The idea of youth and beauty that are linked to being young and thin with a flawless body creates a huge burden for individuals to overcome. In addition, the ways to create these bodies aspire to be achieved by drugs and surgical procedures rather than by adopting a healthy lifestyle involving better nutrition and physical activity. The patient and the healthcare professional must be cautious regarding the drugs and the way that these medications get to be advertised by the drug companies.
Moreover, it is needed to be clarified that the Mediator drug is one of the many drugs examples used for other purposes than they should, while these adverse effects are getting covered under the carpet, leading to both increasing the profit of the companies as well as helping in the conduction of the false body standards and, ultimately, destroying one’s health.
The benefit and well-being of the patient should be the priority of the medical community, as well as, the idea of achieving a desired goal, like improving one’s body image and physique, by working hard and not working less should be the center of the physician’s approach – especially when it comes to dealing with substances that can cost lives. Having the perfect image is not merely comparable to having one’s life and, ultimately, long-term health.




