By Charalampos Karouzos,
Alexander Flemming, Nobel Prize Winner in 1945 for his work on penicillin, is a well-known scientist that discovered Antibiotics, one of the cornerstones of modern medicine. Penicillin as he identified in 1928 has high potency and minimal toxicity in comparison to the antiseptics of his age against bacteria. Although his discovery did not abruptly ignite the interest in further research at the time, it was undoubtedly the first important step to reaching the knowledge available today. However, almost 100 years after the initial discovery of antibiotics we are approaching a critical impasse. Antibiotics known in the past are now useless as resistance from microorganisms has developed and the rate of discovery of novel antibiotics is rapidly decreasing. How far is the post-antibiotic era?
To begin, Antibiotics are chemical substances used to fight off bacterial infections. Bacteria are an important type of living microorganism different from viruses, fungi, and others, capable of causing severe symptoms and even death. Antibiotics, literally meaning “opposing life” translated from Greek, act by inhibiting the development and killing of bacteria that grow in their presence. In other words, bacteria can not tolerate the action of these substances and thus do not cause disease. Antibiotics are produced in nature by microorganisms as a protecting agent against other pathogenic bacteria. Specifically, the first generation of antibiotics was an exact copy of the naturally found ones, for example, Flemming’s Penicillin. Later, a novel form of semisynthetic antibiotics was introduced in which naturally occurring antibiotics were slightly modified chemically to prevent resistance. Further development led to the discovery of full-synthetic antibiotics, preventing resistance and treating more people.
The need to develop more advanced versions of the previous antibiotics arose due to the development of bacterial resistance. In fact, WHO has classified antimicrobial resistance as a “serious threat that is no longer a prediction for the future, it is happening now in every region of the world”. Resistance occurs as new bacterial strains that tolerate current antibiotics, grow via natural selection, thus making the previous generation useless. These new bacterial strains were either unaffected by the presence of the antibiotic or developed enzymes directly destroying the antibiotic (ex. Β-lactamases). Inhibitors of these enzymes proved to be insufficient to provide a definite solution and thus the development of novel antibiotics to treat patients was necessary. However, it was not only the development of such powerful bacteria that led to the situation of today. The effectiveness along with the low cost of the drugs leads to over-usage, accelerating the development of resistance. The medical community and patients saw antibiotics as a panacea to a worldwide health issue that previously devastated humanity, many diseases previously deadly – for instance, the plague pandemic – are now easily treatable with a short course of oral antibiotics.
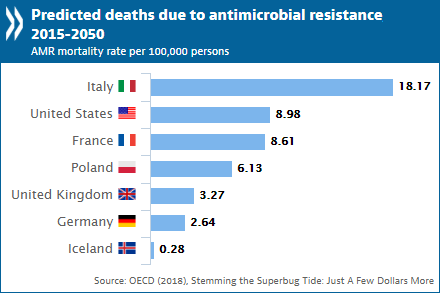
To make matters worse, private pharmaceutical companies, previously the pioneers of novel effective antibiotics development, are seizing funding in the field of research as economic incentives are insufficient. The discovery of substances with antibacterial properties was in the past decades a topic of intensive research doing many steps forward in reaching today’s wide range of available medication. However, the exhaustive research of possible compounds produced by rare microorganisms primarily or as secondary metabolites is not as fruitful anymore. Thus, scientific research has shifted towards non-antibiotic antibacterial therapies including, immunoglobulin therapies, viral phage therapy, fecal microbiota transplant, antisense RNA-based treatments, CRISPR-Cas9-based treatment, and others. Among the most promising of all is a novel approach used by scientists in Boston Massachusetts.
The MIT-based scientific team composed of members of different fields developed a novel antibiotic, Halicin, using a completely new approach in silico (using computers). The crucial difference is based on the processes utilized to formulate the medication, the team focused on the developing field of machine learning and “trained” a computer to identify a chemical substance with the wanted antibiotic properties. To be precise, a deep neural network model was used to predict the growth inhibition of a specific bacterium, E. Coli, a common pathogen in humans. Data were obtained by scanning more than 107 million chemical substances available in chemical libraries giving off several possible candidates categorized by the antibacterial processes. The Artificial Intelligence system that initially discovered several candidate antibiotics was later proved very effective in treating not only the desired bacterium but a wide range of bacteria that in fact are designated by the WHO as very high-priority microorganisms for which antibacterial compounds are needed urgently. In addition, the possible candidates were listed based on a prediction score assessing their threshold of action, availability, and chemical structure.
Halicin, apart from being a very promising antibiotic substance, is a symbol of how the development of new-age technologies can accelerate the treatment available and prevent disease. From the penicillin era until now antibiotics are utilized to prevent bacteria and they have minimized death from previously known conditions. Everything is true but a major issue remains should we base our efforts on fighting off bacterial infection by producing very complicated expensive and unequally distributed medication when other measures must be taken a priori? To be specific, there are three main players on the chessboard. Primarily, individuals must adhere to the doctor’s prescription and not use the drugs if not prescribed to do so. Not only they will be ineffective but they cultivate the ground for antibiotic-resistant bacteria to develop.
Physicians, on the other hand, must prescribe the medication according to the guidelines available and not administer them widely to prevent disease. It seems beneficial for the patient to prevent infection in this manner, however, they are doing more harm than good. Finally, the agriculture industry must limit the usage of medication. It is true that to satisfy the astonishingly high amount of food needed to support the current population, the industry must use such approaches. However, the wide-scale over-usage of the drugs cannot be tolerated in a worldwide plan to fight resistance.

To conclude, it is self-evident that the status quo established in healthcare requires a dramatic change to be able to tolerate the shortage of available antibacterial “weapons” along with the overgrown pan-resistant bacteria. Hopefully, it is not too late to apply the policies needed to bring the issue to a situation that is again manageable. Undoubtedly, modern developments in medicine have been absolutely crucial to provide the current high level of healthcare compared to the situation 100 years ago. In fact, comparing different fields of science one can notice that biology and medicine knowledge has skyrocketed in the last century. However, being dependent on possible future developments in the antibiotics field is a risky decision to take as a failure of the advancements to occur, the price will affect us all inevitably.
References
- Artificial Intelligence Identifies New Antibiotic. new.mit.edu. Available here
- Stopping antimicrobial resistance would cost just USD 2 per person a year. oecd.org. Available here
- Using AI to Find New Antibiotics Still a Work in Progress. directorsblog.nih.com. Available here




