By Frangiska Mylona,
G6PD deficiency may sound like a complex and intriguing name for a disorder. It stands for glucose 6-phosphate dehydrogenase deficiency and in a nutshell, it describes the inability of the glucose-6 phosphate dehydrogenase enzyme to function properly. The symptoms and types of this deficiency are diverse and should be carefully analyzed.
First of all, G6PD deficiency is an X-linked inherited disorder that affects more than 400 million individuals worldwide with the highest prevalence in the Mediterranean sea, the Middle East, Africa, and Asia. The high numbers of prevalence in these areas are attributed to the increased resistance that this deficiency provides against malaria falciparum. More specifically, responsible for the malaria falciparum is a very dangerous parasite called plasmodium falciparum, that in order to develop, it needs the red blood cells of its host. The G6PD deficiency changes the red blood cell morphology providing an extremely hostile environment for its growth!
Responsible for the G6PD deficiency is a mutant form of the enzyme Glucose 6-phosphate dehydrogenase. This enzyme is crucial for the normal function of red blood cells which are quite peculiar types of cells. Their principal function is the transfer of oxygen from the lungs to the tissues of the body and returning the carbon dioxide from the tissues back to the lungs.
Unlike other human cells, their mature form lacks a nucleus and other important organelles like the mitochondria, so in order to survive, they are dependent on the glycolytic pathway and the pentose-6-phosphate pathway which supply them with crucial molecules for their survival like ATP (which stands for adenosine triphosphate and it is an important energy source for the red blood cells) and NADPH (which stands for nicotinamide adenine dinucleotide phosphate) that grants them protection.
Hence, if the glucose 6-phosphate dehydrogenase enzyme is impaired, so the production of the NADPH will also be impaired. NADPH protects the red blood cells from toxic substances like free radicals and reactive oxygen species (ROS) (which are a subtype of free radicals containing oxygen) that could cause oxidative stress. Therefore, if NAPDH production is impaired the oxidative stress can cause damage to the erythrocyte cell wall leading to the cell’s premature death. Also, the glucose 6-phosphate dehydrogenase enzyme is responsible for the production of antioxidants like Glutathione.
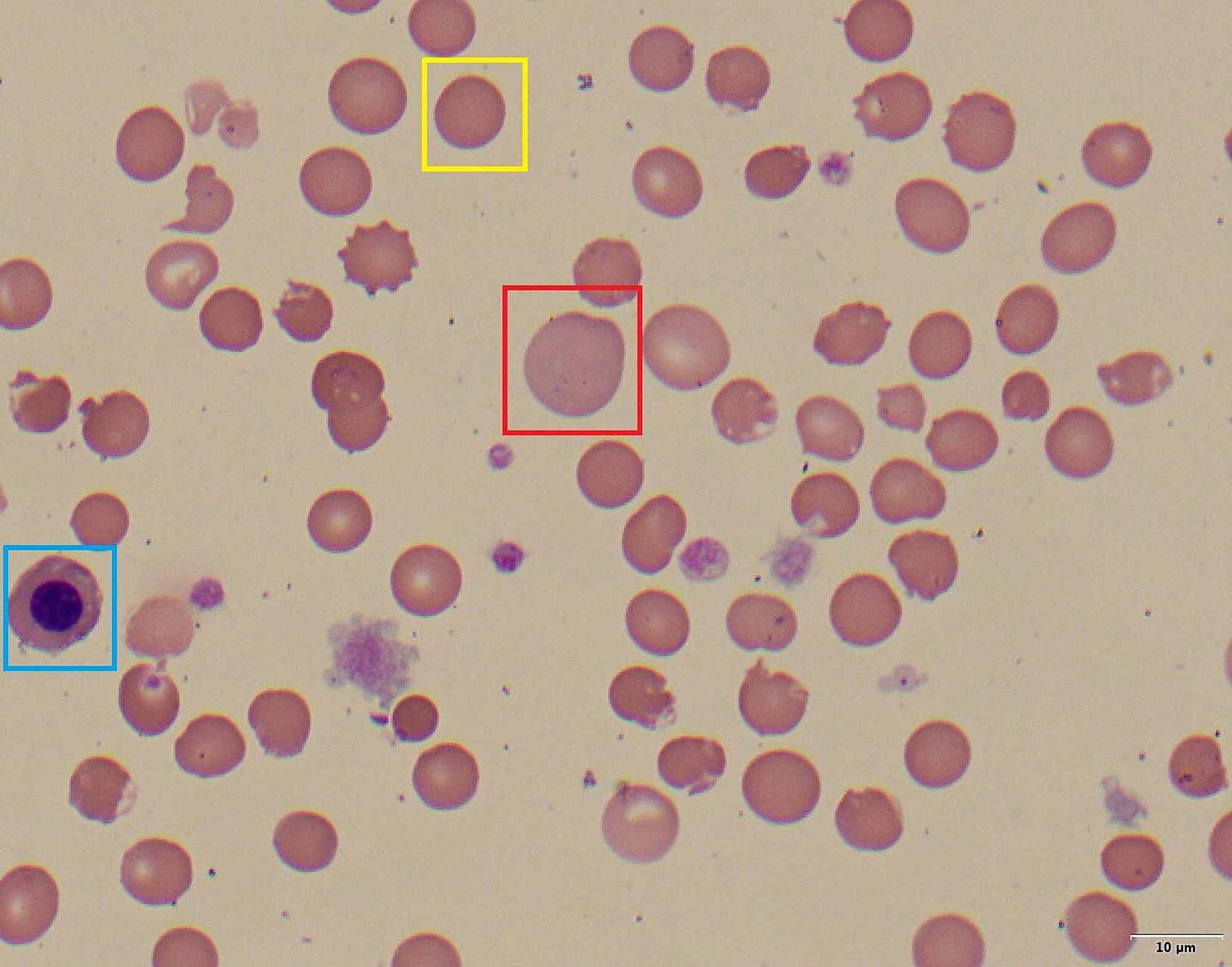
Glutathione protects the cell against oxidative damage from ROS, and free radicals and helps the hemoglobin to keep its normal state. If proteins like hemoglobin get oxidized, they can create insoluble masses that get attached to the red blood cell’s membrane. These masses are called Heinz bodies. This results in a rigid state of the red blood cells which are removed more rapidly from the circulation by the liver and spleen.
Therefore, the major complications of this disorder are severe hemolytic anemia, which can be acute or chronic, since the cells can not get detoxified and also, and neonatal jaundice.
One more interesting fact about this disorder is the fact that due to its inheritance pattern it affects mostly males than females. As it is mentioned above, it is inherited in an X-linked way. The etiology of this mechanism is explained by the fact that the gene responsible for the formation of the G6PD enzyme is located on the X chromosome.
Normally, the human genome contains 46 chromosomes that are organized in 23 pairs, where the 22 pairs are the same for both sexes, while the 23rd pair determines the biological sex of the human embryo. In biological males, the 23rd pair of chromosomes include one X and one Y chromosome, meaning that they are hemizygous for the X-chromosome. Hemizygous is a term used for a person that usually has one chromosome of the chromosome pair. This is more applicable for males when it comes to the X-chromosome.
On the other hand, in biological females, the 23rd pair contains two X chromosomes. The Y-chromosome is the determinant for the biological sex of each human and if it is present in the human genome, the person is a biological male and on the other contrary, if it is absent the person is a biological female. The Y-chromosome is inherited from father to son. Also, one important concept is the alleles. Alleles are the alternative forms of a gene positioned on each of these 22 pairs of chromosomes while the X-linked alleles are the genes located only on the X-chromosomes therefore they exist in two copies in the biological females (XX) and in one copy in the biological males (XY).
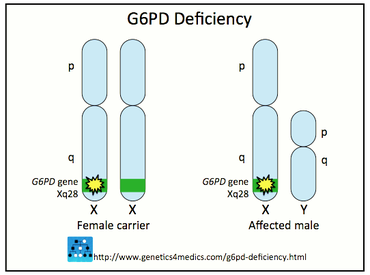
From all of the above, we understand that females can either be unaffected, heterozygous, or homozygous for this deficiency while males are either hemizygous or unaffected which leads to the conclusion that they will either have the disorder or they will be healthy. The heterozygous females can present an ‘’erythrocyte mosaicism’’ because in each of their cells only one X-chromosome can be activated, while the other X-chromosome is inactivated. This mechanism is a thoughtful process of nature so that females will have one functional X-chromosome in each body cell like the males, in order not to have more genetic material than them. Take into consideration that the X-chromosome is bigger than the Y-chromosome and subsequently it carries more genetic material.
Moreover, there is a broad spectrum of mutations for this gene with more than 400 of them to have been identified providing numerous variants to the disorder. Usually, the mutations are missense mutations (meaning that the normal sequence for the G6PD with the mutated one can differ by only one nucleotide) while large mutations like deletions can cause a complete absence of the G6PD which can be lethal.
Due to the number and severity of mutations, we classify the G6PD deficiency into five classes. These classes of G6PD have to do with whether there is some enzymatic activity or not and therefore their severity is attributed to the residual enzymatic activity.
1) Class 1; very severe deficiency causing a severe chronic chemolytic deficiency.
2) Class 2; severe deficiency with episodic hemolytic anemia. An example is the Mediterranean G6PD deficiency with decreased enzymatic activity.
3) Class 3; moderate anemia, example is G6PD-A ̄ deficiency.
4) Class 4; no clinical symptoms
5) Class 5; overproduction of G6PD, mostly being asymptomatic.
Although the deficiency can cause no symptoms, there are some factors that along with this disorder, can evoke hemolytic anemia like:
1) AAA; Some oxidant drugs can be dangerous for people with this disorder. This acronym stands for some antibiotics, antipyretics, and antimalarials that should be avoided.
2) Favism; Fava, this tasty bean can cause hemolytic anemia, especially in people with the Mediterranean variant!
3) Infection; Infection can cause oxidative damage by producing some free radicals, which diffuse into the red blood cells that, due to the deficiency, cannot be properly protected.

It should be mentioned that G6PD deficiency does not only affect the red blood cells but every cell in the organism. Yet, it is more severe in the erythrocytes because they are solely dependent on the pentose-6-phosphate pathway to produce the NAPDH so that they will be protected against oxidative stress, unlike other tissues that have alternative sources for NAPDH production.
About the diagnosis of this disorder:
Diagnostic tests like the quantitative spectrophotometric analysis or rapid fluorescent spot test detect the generation of NADPH. The positive result is confirmed if the blood spot fails to fluoresce under ultraviolet light, however, this test may be unsuccessful in patients with acute hemolysis or in heterozygous females. Another factor that helps in the diagnosis is the positive family history for this deficiency. Additionally, in our days, prenatal diagnosis is possible by PCR amplification and restriction of endonuclease digestion.
In conclusion, as it is already understood this deficiency can be asymptomatic and the affected person may not know if they are suffering from it. However, the family medical history should be an alarming factor in this deficiency, and if someone is suspecting that they might be suffering, they should get tested and avoid all the possible hemolytic factors that were mentioned above.




